Polythiophene as a sensor model for chlorofluorocarbon, Fluorine and Oxygen gas using DFT Calculations [Completed]
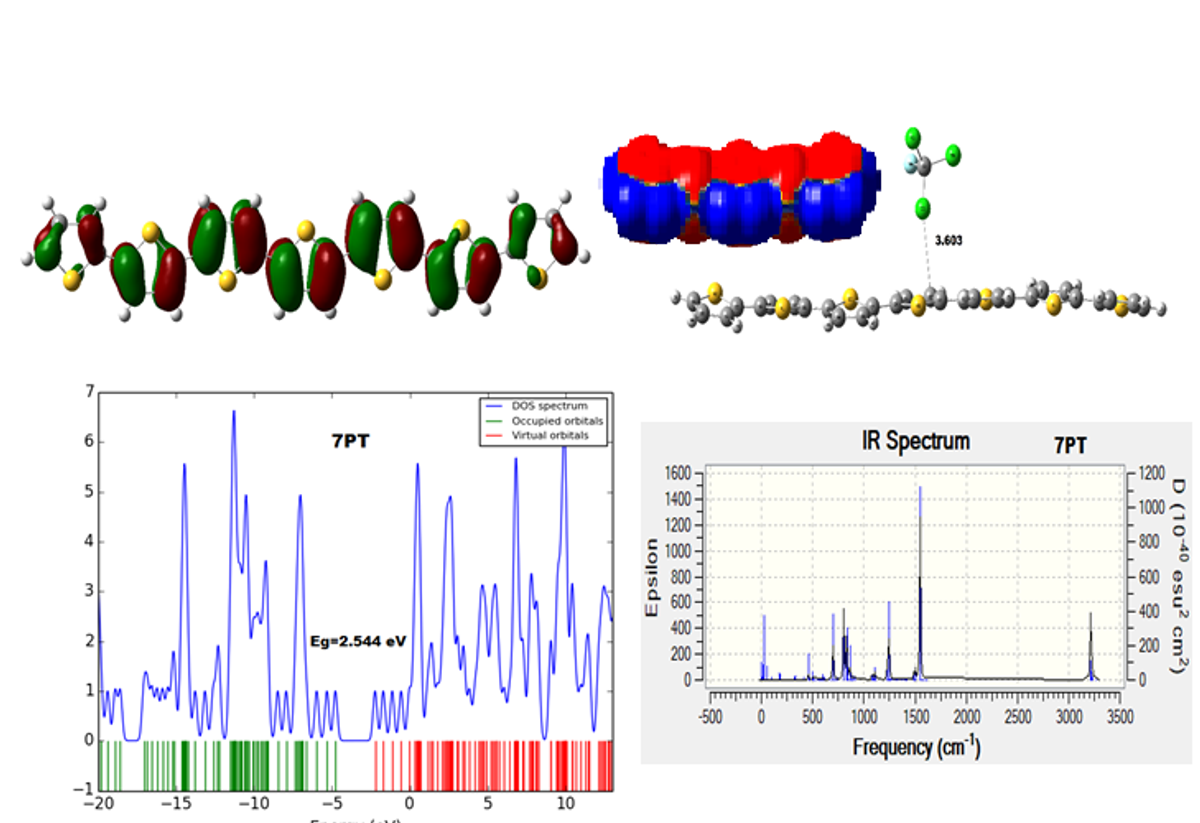
Adsorption of CFC-11, CFC-12, CFC-13, F2 and O2 has been investigated systematically on the polythiophene (PT) moieties through density functional theory (DFT) calculations at the B3LYP/6-31G (d) level. Here, geometry optimizations have been performed on a number of polythiophene (5 and 7) complexes. Likewise, adsorption energies, dipole moments, HOMO-LUMO orbital analysis, density of states (DOSs) and global indices, are calculated using the DFT method. Energies of interaction and HOMO-LUMO gap show that polythiophene has high sensitivity towards CFCs (CFC-11, CFC-12 and CFC-13), F2 and O2 for B3LYP functional. Based on our results, it is found that CFCs, F2 and O2 molecules can be physically adsorbed on the moiety of polythiophene.

